e_AmmoniaSynthesis
Electrolytic synthesis of ammonia from water and nitrogen under atmospheric pressure
The production of NH3 is crucial since it is an essential ingredient in the manufacture of fertilizer and many important chemicals. Its distributed use, including the selective catalytic reductant of NOx emitted from ships and stationary facilities, CFC alternatives, and safe and economical energy carriers (transport and storage media) for future energy systems will also increase.
However, at present, almost all NH3 is synthesized by the Haber-Bosch process on a large-scale at high temperature and high pressure. For its distributed use, a new process must be created that can be operated at a lower synthesis temperature and pressure. Furthermore, we are currently facing another problem: since we must reduce the amount of CO2 emissions, we have to create a new ammonia synthetic process that can be operated without such emissions.
To meet these requirements, we proposed the direct electrolytic synthesis of NH3 from H2O and N2 under atmospheric pressure using molten salt electrolytes, and we are currently developing a process that is directed toward industrialization.
Its principle is shown in Fig. 11.
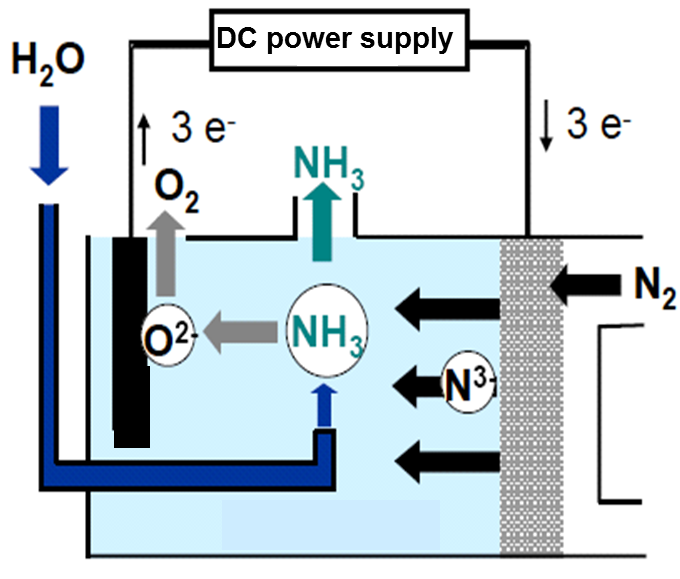
Cathodic reaction: 1/2 N2 + 3 e– → N3-
Gas-liquid reaction: N3- + 3/2 H2O → NH3 + 3/2 O2-
Anodic reaction: 3/2 O2- → 3/4 O2 + 3 e–
Total reaction: 1/2 N2 + 3/2 H2O → NH3 + 3/4 O2
Fig. 11 Principle of electrolytic NH3 synthesis from water and nitrogen
Nitride ion (N3-) is produced in an electrolyte by a cathodic reduction of N2 gas. When water vapor is supplied to the electrolyte, N3- and H2 O react to produce NH3 and O2- : N3- + 3/2H2 O → NH3 + 3/2O2-. The produced oxide ion (O2-) is anodically oxidized at the anode to evolve O2 gas. The overall reaction is expressed as 1/2N2 + 3/2H2O →NH3 + 3/4O2
The standard theoretical electrolysis voltage corresponding to the total reaction is calculated as 1.17 V at 600K. The operation data of bench-scale electrolytic cells show that the process has promising practical aspects.
Since the conventional Haber-Bosch process utilizes natural gas as a hydrogen source, it emits a large amount of CO2. The experimental data of bench-scale experiments with estimation results based on economic aspects encourage us to propose an ammonia energy system whose concept is shown in Figs. 12 and 13.
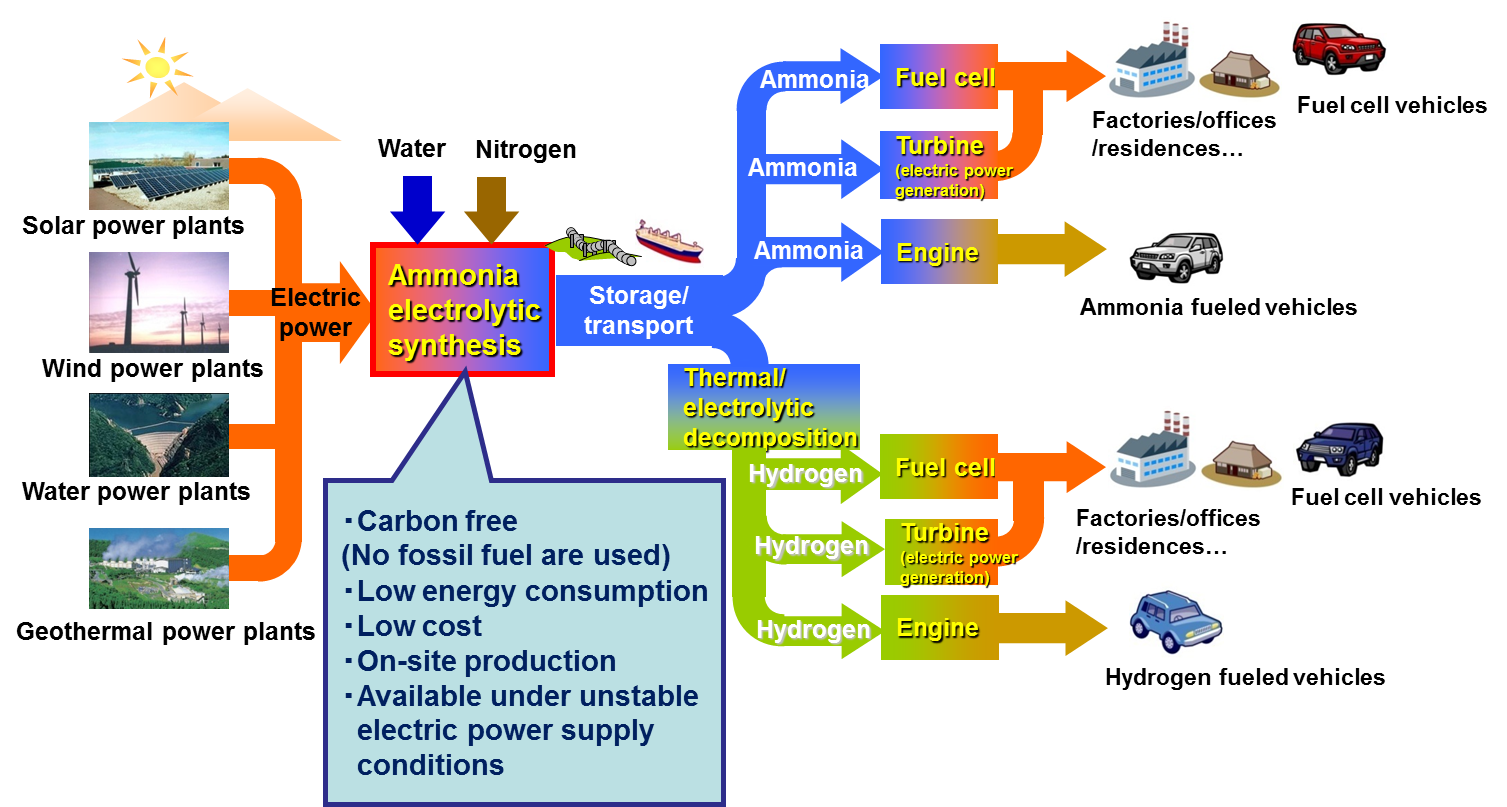
Fig. 12 Expected role of our electrolytic process in ammonia energy system
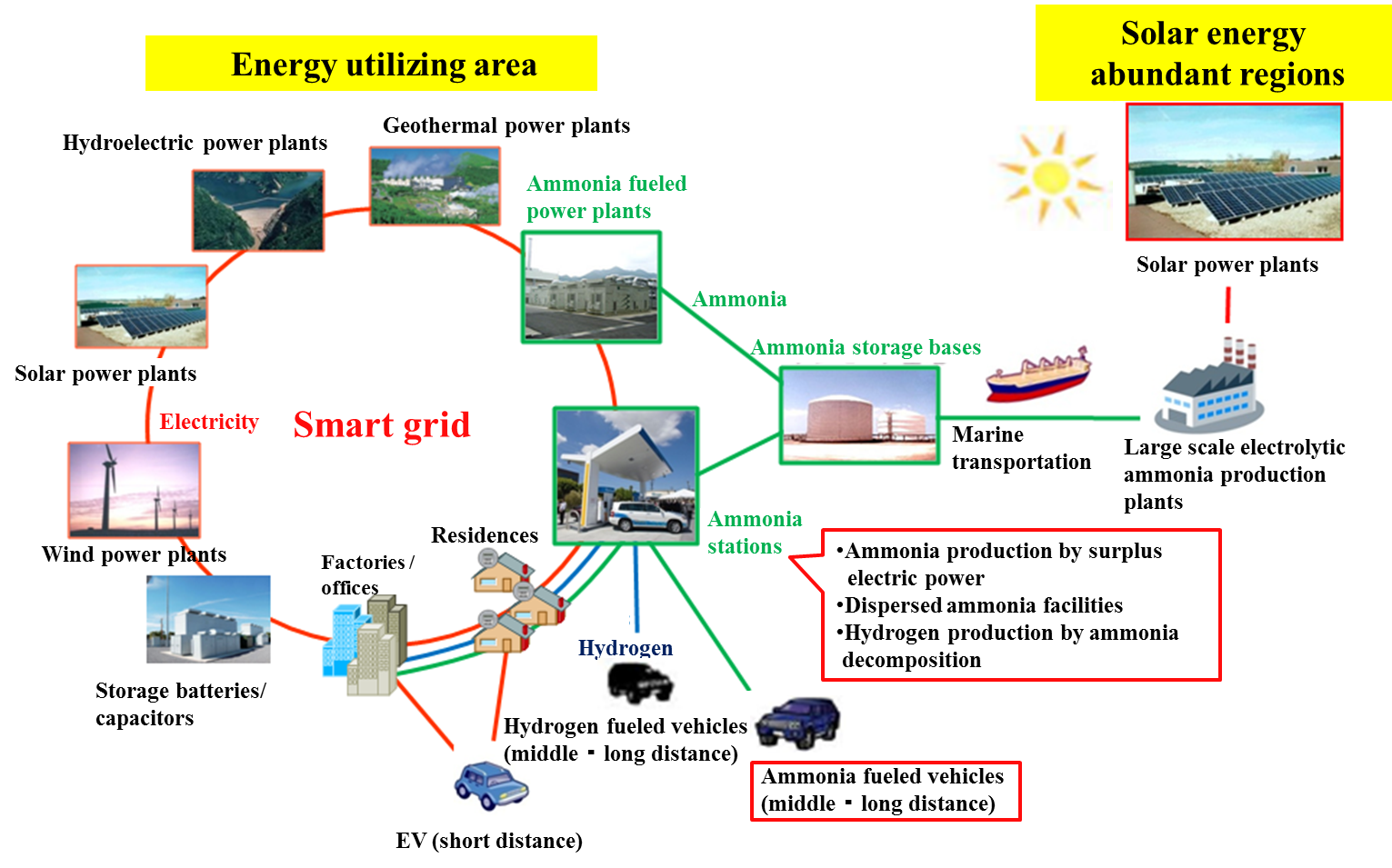
Fig. 13 Overview of ammonia energy system
When we consider the role of this process from the aspects of reducing CO2 emissions, we will be able to provide an effective solution to a serious complicated problem, which is composed of two incompatible factors: food shortages and increasing CO2 emissions.
